Electronics and communications industry uses large amounts of tin-plated copper wire, tin-plated copper production and during use, inevitably produce large amounts of waste tinned wire, tin-plated copper wire of this process, except for a part of the production outer bronze alloy, the use of other applications, require tin layer on the copper removal. Therefore, the selection of a suitable de- tinning process to achieve less investment, easy operation, good tin removal effect, less copper loss, is very important to improve efficiency.
First, the chemical law
Chemical de-tinning is to use the difference in chemical activity between tin and copper to design the process to achieve the purpose of removing tin and retaining copper. In line with the principle of environmental protection, this article does not introduce the tin removal process of cyanide system.
(1) Acid system
![]()
The process is operated at room temperature, the process is relatively simple, but the corrosion of copper is more, generally not used.

The process is simple to operate, and the tin is removed at room temperature, and the raw materials used are relatively cheap, and can be referred to.

At room temperature operation, the glacial acetic acid used in the process is relatively expensive and used with caution.

Operating at room temperature, the process is simple, and the addition of hydrogen peroxide should be careful to prevent copper from being oxidized.
(2) Alkaline system
Sodium hydroxide (salt alkali) 160 ~ 180g / L
Anti-staining salt S70~80g/L
Sodium citrate 15g/L
Second, the electrochemical method
The method of electrochemical deplating can effectively remove the tin plating layer, and the deplating equipment includes: drum deplating, basket deplating, etc., and the deplating solution has an acidic system and an alkaline system.
(1) Alkaline system: sodium hydroxide deplating solution, content 135g / L, temperature 80 ~ 90 ° C, voltage 1 volt. The deplating solution needs to be heated.
(Ii) an acidic System: fluoroboric acid or fluosilicate bath retreat system, at a concentration of about 150g / L, room temperature, about 1 volt potential, does not attack the copper to prevail.
As long as the process conditions are properly controlled, the electrochemical deplating method can not etch the copper matrix, and can recover the sponge tin. After washing, it can be smelted and removed to obtain 99.9% tin. It can be used to prepare tin alloy such as solder or used for producing tin. Chemical Products.
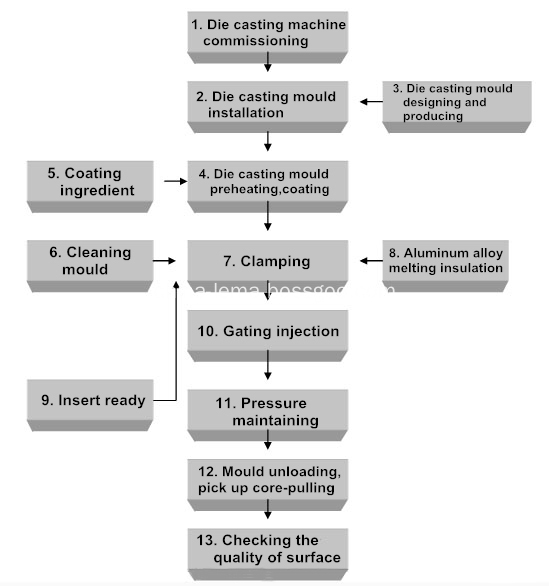
The die casting part production flow chart
Injection speed of die cast process
|
Pouring liquid metal tie up chamber volume percent |
Injection speed(cm/s) |
|
≤30 30-60 >60 |
30-40 20-30 10-20 |
High speed computing formula
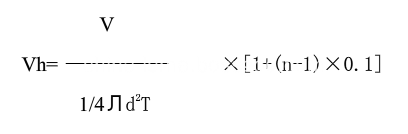
V -------------Cavity volume (CM3);
N ------------ Cavity No;
D ------------ Die-casting drift (CM);
T ------------- Proper filling time.
The production of aluminum and Zinc Alloy Die Casting parts.

A380 Aluminum Die Casting, Aluminum Die Cast Part, Die Casting Parts, Zinc Die Casting
NINGBO BEILUN LEMA MACHINERY TECHNOLOGY CO.,LTD , http://www.china-lema.com